The LiquiBand FIX8® Open
What is LiquiBand FIX8® Open device?
The LiquiBand FIX8® Open Hernia Mesh Fixation device is designed for the application of cyanoacrylate adhesive to an implanted hernia repair mesh, in order to fix the mesh to the underlying tissue and to hold closed easily approximated wound edges closed. The device consists of:
a) n-butyl-2-cyanoacrylate adhesive
b) a delivery instrument which dispenses the adhesive
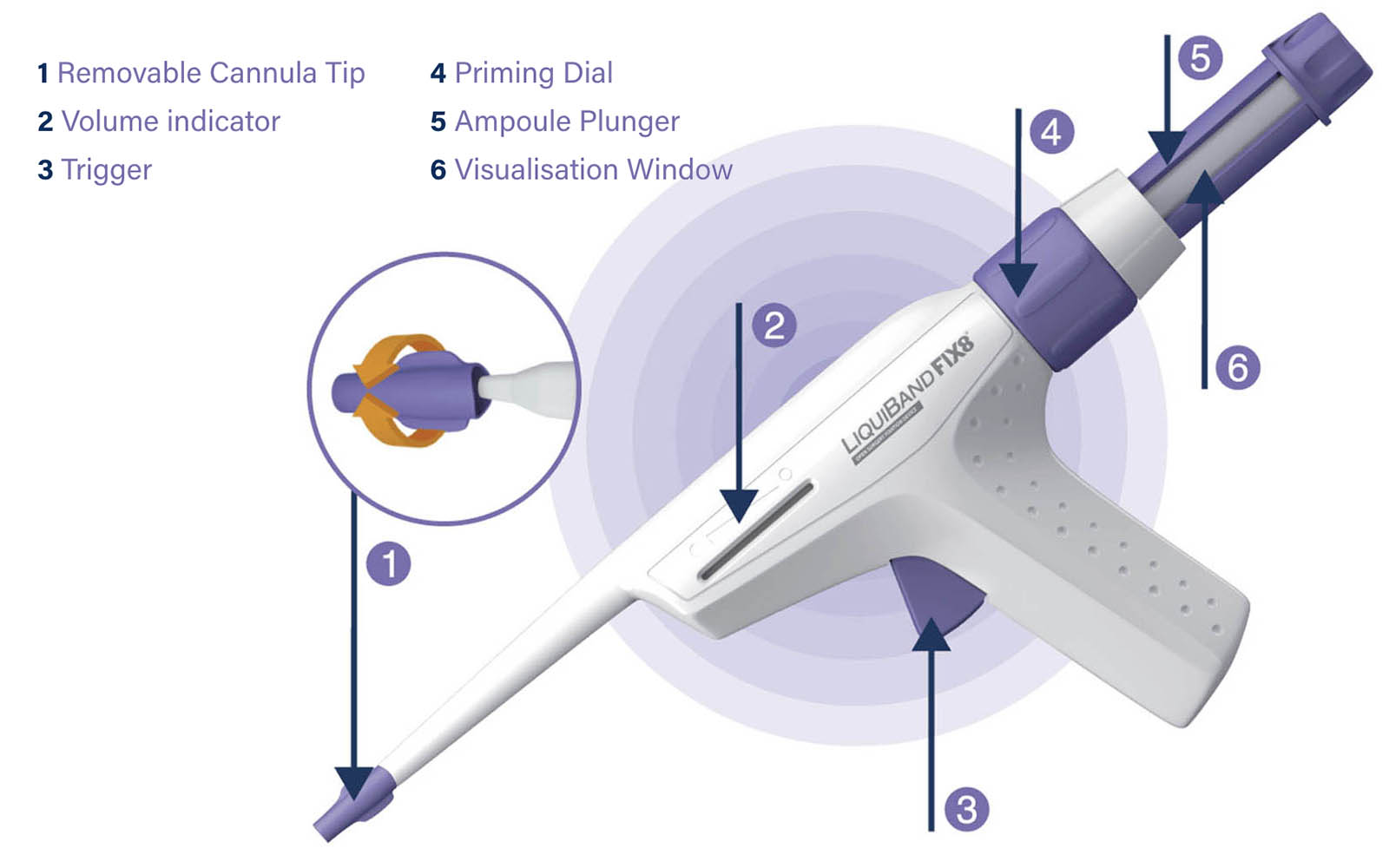
Material/substances in contact with patient tissues:
The implanted material contains ≥99.5% n-butyl-2-cyanoacrylate adhesive. The cyanoacrylate adhesive bonds the hernia mesh to the tissue.
What is the role of the device in hernia mesh surgery?
The LiquiBand FIX8® Open Hernia Mesh Fixation device is intended for use in open surgical repair of inguinal and ventral incisional hernias, achieved through the fixation of prosthetic mesh to the abdominal wall and to hold easily approximated skin edges of wounds from the hernia repair incision.
Kind of patient the device is intended to be used?
The target population of the LiquiBand FIX8® Open Hernia Mesh Fixation device is for use on patients who are having their hernias treated through prosthetic mesh fixation.
Use of prosthetic mesh for hernia repair should be in alignment with the indications of the prosthetic mesh.
The target population of the device has been appropriately reflected in the indications and contraindications:
- Indications:
• The LiquiBand FIX8® Open Hernia Mesh Fixation device is intended for use in open surgical repair of inguinal and ventral incisional hernias, achieved through the fixation of prosthetic mesh to the abdominal wall and to hold closed easily approximated skin edges of wounds from the hernia repair incision.
- Contraindications:
• The device is not intended for use when prosthetic material fixation is contraindicated.
• Do not use on patients with a hypersensitivity to cyanoacrylate adhesives or formaldehyde.
• Do not use for the fixation of meshes constructed with polytetrafluoroethylene (PTFE) or absorbable materials.
• Do not use device for closure or fixation of cerebral tissues, blood vessels or peripheral nerves.
• Do not use on any wounds with evidence of microbial, bacterial or fungal infections or gangrene.
• Do not use topically on mucosal surfaces or across mucocutaneous junctions or on skin which may be regularly exposed to bodily fluids.
• Do not inject intravascularly or ingest.
• Do not use topically on decubitus ulcers, animal or human bite wounds, or stab wounds.